Phase 2™ carb controller is a proprietary, natural ingredient derived from the white kidney bean. Phase 2™ is not a stimulant; it works by reducing the enzymatic digestion of dietary starches. Phase 2™ has been clinically shown to reduce the digestion and absorption of dietary starches by up to 66%.
Phase 2™ carb controller has been extensively studied; more than 14 clinical studies have been conducted in the past 15 years. Phase 2™ is a market leader, and has been used successfully in multiple products worldwide.
Phase 2™ white kidney bean extract has been reviewed by U.S. FDA to permit structure/function claims:
Additionaly, in its monograph for white kindey bean extract, Natural Health Products Directorate of Canada permits the following claim for weight management: 3000 mg per day standardized to 3000 AAIU per gram : assists in weight management when taken before meals.
Phase 2™ carb controller has also been shown to have efficacy for pets. 88% of dogs in a multi-clinic study lost weight.
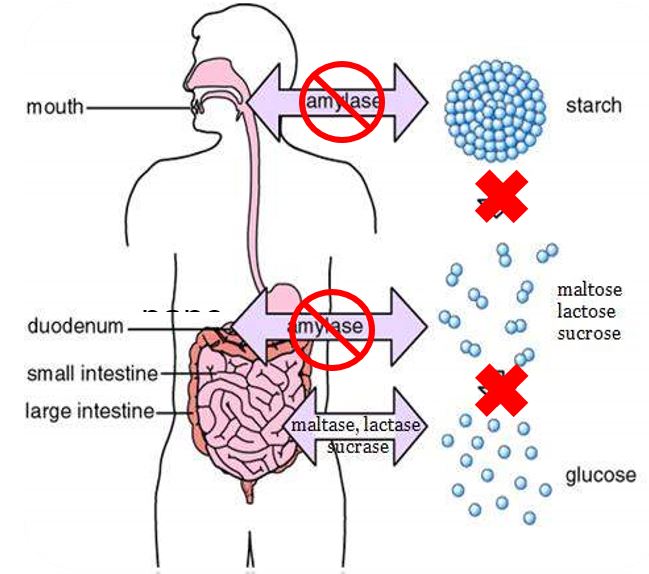
It works on the starchy, white component of carbohydrates selectively without affecting the digestion of healthy carbs such as fruit and whole grains. It may also play a role in regulating blood glucose already in normal limits.
Phase 2® inhibits both salivary and pancreatic α-amylase, reducing the digestion and absorption of carbohydrate, thereby suppressing:
However, a word of caution: all starch blockers are not created equally. While Phase 2 has years of substantial research behind it, other “so called” starch blockers or white kidney bean extract have no research basis at all. Before selecting any product, check to see if adequate research and safety studies have been conducted to make sure you’re getting the real thing.
TWO Approved US FDA Functional claims
Phase 2® is the first nutritional ingredient with two permitted structural / functional claims by US FDA:
Other Phase 2® accolades include:


The latest (published Apr 2018) systematic review & meta-analysis on the effectiveness of Phase 2® in weight loss and reducing body fat produced the following findings:
More than two dozens of clinical studies with more than 600 subjects have assessed and confirmed the safety and efficacy of Phase 2® Carb Controller in glycemic control and weight management.
Vinson, J. A., Kharrat, H. A., & Shuta, D. (2009). Investigation of an amylase inhibitor on human glucose absorption after starch consumption. The Open Nutraceuticals Journal, 2(1).
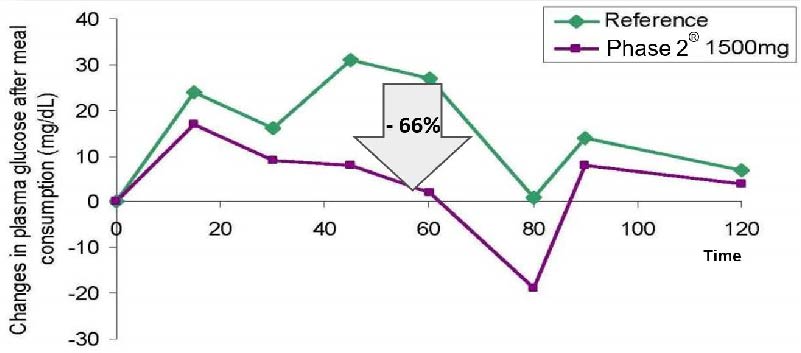
In a study conducted in University of Scranton (USA), 11 fasting healthy subjects consumed 1500mg of Phase 2 or placebo, together with 4 slices of bread (60g carbohydrate). Subjects’ blood glucose level was measured before and after meal periodically every 15 mins up to 2 hours. Phase 2® resulted in reduced postprandial glucose response by 66% in 1st hour.
Grube, B., Chong, W. F., Chong, P. W., & Riede, L. (2014). Weight reduction and maintenance with IQP‐PV‐101: A 12‐week randomized controlled study with a 24‐week open label period. Obesity, 22(3), 645-651.
In the recent most comprehensive human study conducted involving 123 healthy participants, subjects taking a proprietary extract of the white bean, Phase 2 Carb Controller® lost an average of seven pounds more than those on placebo after 12 weeks. In addition, 73.5% of the participants in the weight management phase successfully maintained their body weight after 24 weeks.
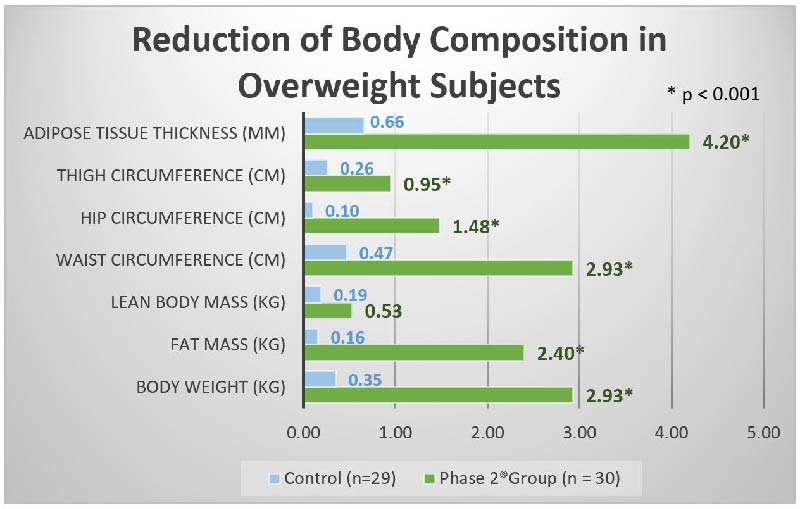
Celleno, Leonardo, et al. "A dietary supplement containing standardized Phaseolus vulgaris extract influences body composition of overweight men and women." International journal of medical sciences 4.1 (2007): 45.
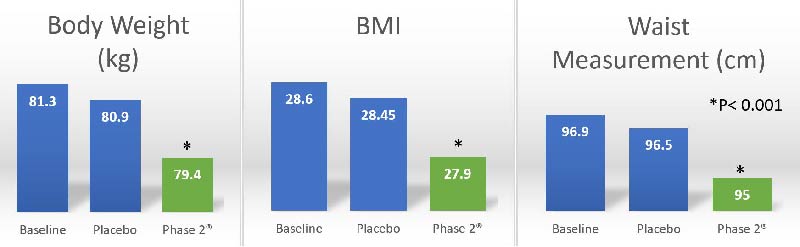
A clinical study was conducted by researchers in Celleno University of Rome, Italy on conducted on 60 overweight volunteers. Subjects were given either one 445mg of Phase 2® supplement or a placebo pill daily for 30 consecutively days before a carb-loaded meal. Various body compositions of these subjects were measured after 30 days.
Subjects receiving Phase 2® supplement with a carbohydrate-rich (2000- to 2200-calorie diet) had significantly (p less than 0.001) greater reduction of body weight, BMI, fat mass, adipose tissue thickness, and waist/hip/thigh circumferences while maintaining lean body mass compared to subjects receiving placebo.
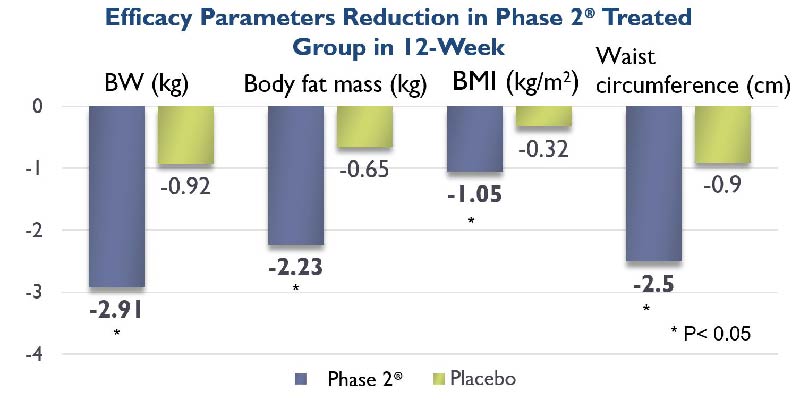
Perricone, Nicholas V. "Enhanced weight loss from a dietary supplement containing standardized Phaseolus vulgaris extract in overweight men and women." METHODS 12.13 (2010): 15-22.
A randomized, double blinded, and placebo-controlled investigation was carried out on 101 subjects by researchers in Zhejiang University, Hangzhou, China. Subjects were given either 1,000 mg Phase 2® or placebo 15 minutes before each meal for 60 consecutive days.
Body weight, waist and hip circumferences, and blood chemistry values were measured at the beginning, at 30 days, and at the end of the 60-day treatment phase. The results showed very significant (p less than 0.001) reduction of body weight, BMI and waist circumference.